
Precision Medicine
in Oncology
We have a game changer !

50% of patients are missing the best therapy due to insufficient molecular testing.
Precision medicine is evolving very fast, 40% of drug approvals in oncology of the 21st century are for biomarker-defined populations, however :
- In NSCLC, 50% of patients are missing the best therapy due to insufficient molecular testing.
- Only 7% of patients do enroll in clinical trials while 70% are willing to, and 83% of oncologists agree that it would have been beneficial for patients to be enrolled.
- Access to molecular testing and precision medicine is highly unequal both within and across developed countries due to high cost and infrastructure limitations (Mateo et al., 2023).

Emerging trends in drug development

The number of products under development in oncology has grown significantly over the last decade, with more than 2,000 products currently under development.
The demand for precisely characterized populations is increasing, mostly based on molecular biology techniques.
The demand for precisely characterized populations is increasing, mostly based on molecular biology techniques.
Our digital pathology algorithm for early tumour characterisation by virtual molecular biology predicts:
+1’000
DNA & RNA alterations
21
Cancer types
Reduction of time
Selection of patients most at risk for emergency molecular testing.
Remove unnecessary tests
Reduction by half of the number of tests through the selection of appropriate molecular tests.
Enlargement of the recruitment pool for the clinical trials
Identification of the most relevant clinical studies on D0 for patients based on its eligibility criteria.

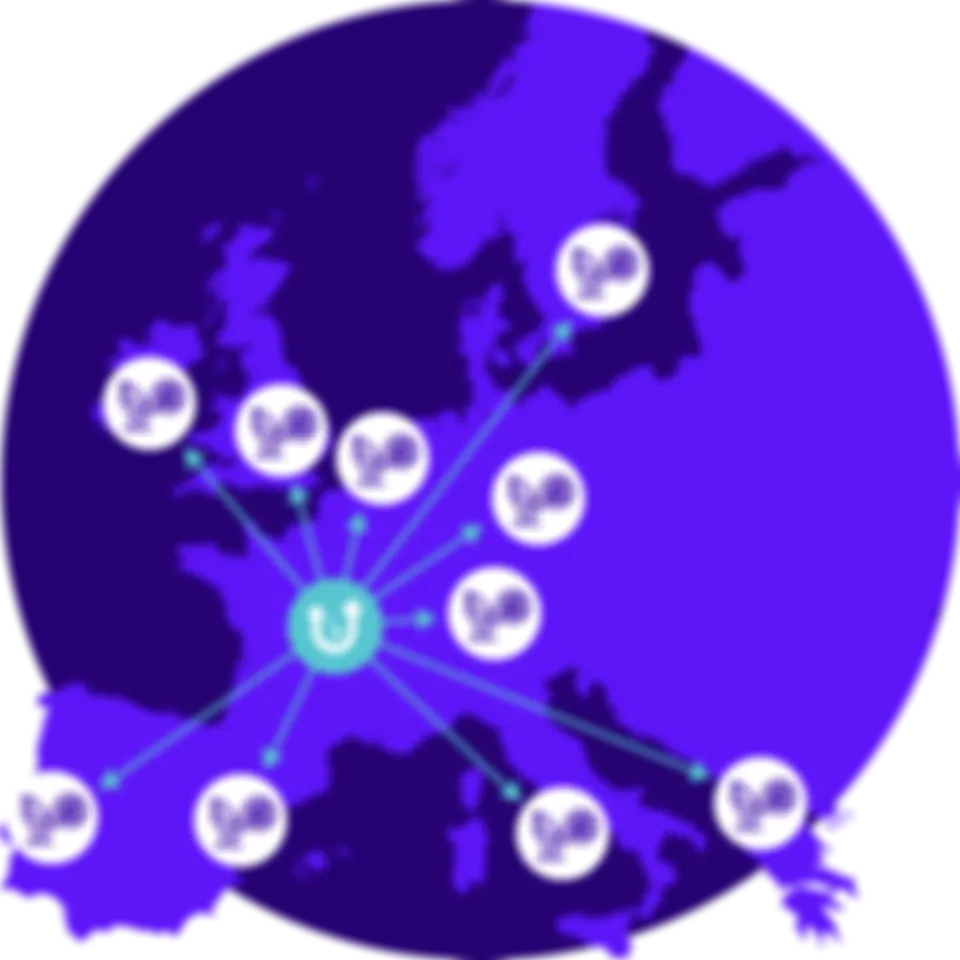
Distributed & targeted recruitment solution
CROs register their study into the Ummon Crawler CRO interface.
The study is added to our Clinical Trial Matching System and dispatched across our partner labs.
Ummon Crawler will predict molecular biomarkers, then hits are generated when patient characteristics match your clinical study.
A report is sent to the clinician, patient consent is then collected and clinician contact is sent to the CRO.
A predictive patient matching system

The tissue sample of the patient is sent to the pathologist.

Chara-prAIdict predicts molecular biology from the tissue and Ummon Crawler matches with a large dataset of clinical trials.

A report with best clinical trial opportunities is sent to the clinician.

The clinician with the patient decide if they accept further characterization to fill eligibility criteria, leading to gold standard healthcare.
Our Technology
Revolutionary Dual-Model Analysis
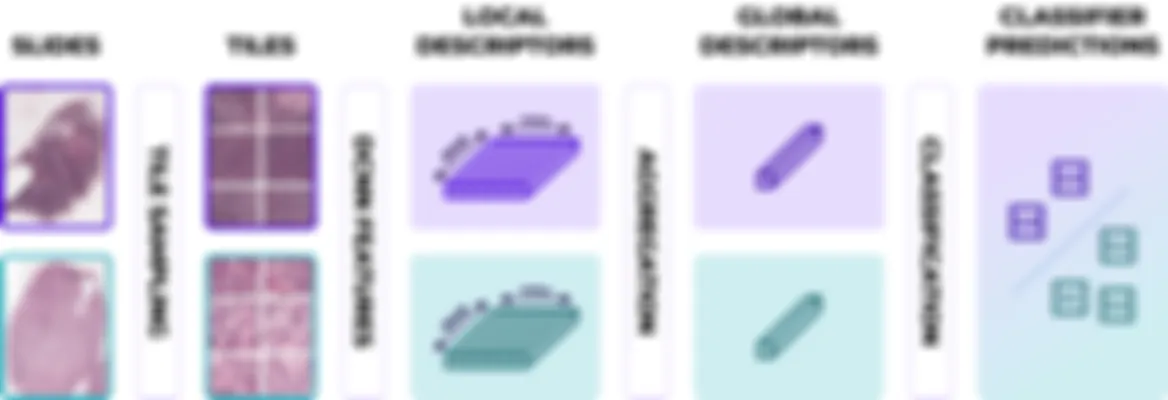
Chara-prAIdict features an innovative scoring system blending cutting-edge deep learning with contextual molecular pathways, ensuring performances and fine-grained predictions for high precision diagnostics. (Valderrama et al., 2024)

Advanced Calibration for Consistency
Our unique calibration module overcomes interlaboratory and interscanner variations. Using a calibration process aligned with our extensive databases, we ensure consistent, accurate results, enhancing reliability (Dumas et al., 2022).
Intuitive User Experience
Chara-prAIdict’s ergonomic design allows even first-time users to confidently manage the system, enabling quick and efficient analysis without compromising accuracy. Ideal for seamless integration into medical professionals’ diagnostic workflow.

History of our technology
2018 - Coudray et al.
2018 - Coudray et al.
Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning.
Nat. Med.
2020 - Fu et al.
2020 - Fu et al.
Pan-cancer computational histopathology reveals mutations, tumor composition and prognosis.
Nat. Cancer
2021 - Howard et al.
2021 - Howard et al.
The impact of site-specific digital histology signatures on deep learning model accuracy and bias.
Nat. Commun
2021 - Van der Laak et al.
2021 - Van der Laak et al.
Deep learning in histopathology: the path to the clinic.
Nat. Med.
2022 - Dumas et al.
2022 - Dumas et al.
Inter-Semantic Domain Adversarial in Histopathological Images.
ArXiv
2023 - Morel et al.
2023 - Morel et al.
Preliminary evaluation of deep learning for first-line diagnostic prediction of tumor mutational status.
sci. rep.
2023 - Filiot et al.
2023 - Filiot et al.
Scaling Self-Supervised Learning for Histopathology with Masked Image Modeling.
sci. rep.
2024 - Valderrama et al.
2024 - Valderrama et al.
Breast-NEOprAIdict: a deep learning solution for predicting pathological complete response on biopsies of breast cancer patients treated with neoadjuvant chemotherapy.
In review
2024 - Morel et al.
2024 - Morel et al.
MultiVarNet: A Deep Learning and Label Engineering Approach for Predicting Tumour Mutational Status at the Protein Level.
MICCAI 2024
A unique positioning
For Clinical Research
Economical
Cut the cost of your patient recruitment strategy by a factor of 3.
Expanding patient recruitment
Accelerate your clinical studies by expanding your patient recruitment pool.
For healthcare
Free
Unlike most digital pathology solutions (~25€/click on average), slowing down their deployment.
Additional Patient testing
Increase in the number of useful tests with the indication of rare panels currently not carried out and the molecular testing by manufacturers wishing to recruit patients.
Optimize social security cost
- With PCR: we save €152/patient with NSCLCs.
- With NGS: Our solution makes it possible to screen twice as many people for the same number of tests (Morel et al., 2023).
Game changing moment
Technology validated
Our AI pipelines have been validated in high quality peer reviewed journals and beta testing.
Consortium constructed
A consortium with 20 pathology labs, 5 partners, both in private and public sectors with European counterparts, has been created.
Tailored proposal
We look for strategic partners that will help us tailor our process to enhance their recruitments.
- The first step will be to work with our partnered labs, define the process implement it by hand,
- then plug-in our solution for your offers.